
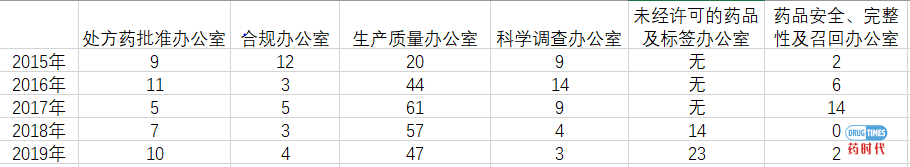
1、Warning Letter 2015
https://www.fda.gov/drugs/warning-letters-and-notice-violation-letters-pharmaceutical-companies/warning-letters-2015
2、Warning Letter 2016
https://www.fda.gov/drugs/warning-letters-and-notice-violation-letters-pharmaceutical-companies/warning-letters-2016
3、Warning Letter 2017
https://www.fda.gov/drugs/warning-letters-and-notice-violation-letters-pharmaceutical-companies/warning-letters-2017
4、Warning Letter 2018
https://www.fda.gov/drugs/warning-letters-and-notice-violation-letters-pharmaceutical-companies/warning-letters-2018
5、Warning Letter 2019
https://www.fda.gov/drugs/warning-letters-and-notice-violation-letters-pharmaceutical-companies/warning-letters-2019
历史上首次!FDA局长就数据可靠性发表评论

四场集中直播,一个核心主题!——前FDA检查官Peter Baker先生为您解读数据可靠性
生产过程中的数据可靠性与风险管理
Data Integrity and Risk Management in Manufacturing Operations
电脑软件保证和数据可靠性:一个基于风险的方法
Computer Software Assurance & Data Integrity: A Risk-Based Approach
数据可靠性和色谱:数据管理方法
Data Integrity & Chromatography:A Data Governance Approach
欢迎您点击下方【阅读原文】参加直播!
⬆️点击上方图片,了解更多信息


发布者:药时代,转载请首先联系contact@drugtimes.cn获得授权


 为好文打赏 支持药时代 共创新未来!
为好文打赏 支持药时代 共创新未来! 
